What Is An Elementary Reaction In Kinetics
Elementary rate laws Chemical kinetics Elementary reaction
Molecularity of Elementary Reactions - Chemical Kinetics - Chemistry
Q ):- assuming an elementary reaction h2o2+3l-+2h+→2h2o+l3- the effect Reaction reversible rate law kinetics chemical Chemical kinetics presentation
Chemical kinetics: 2 molecularity elementary reaction
Difference between elementary and nonelementary reactionKinetics reaction slideshare General chemistry chemical kinetics: mechanisms. free in-depth study guideReaction kinetics.
Reaction chemical kinetics mechanisms ppt powerpoint presentation overall stepKinetics reaction formulas concentration Reaction kinetics order reactant chemist mechanism fractional respectChemical kinetics.
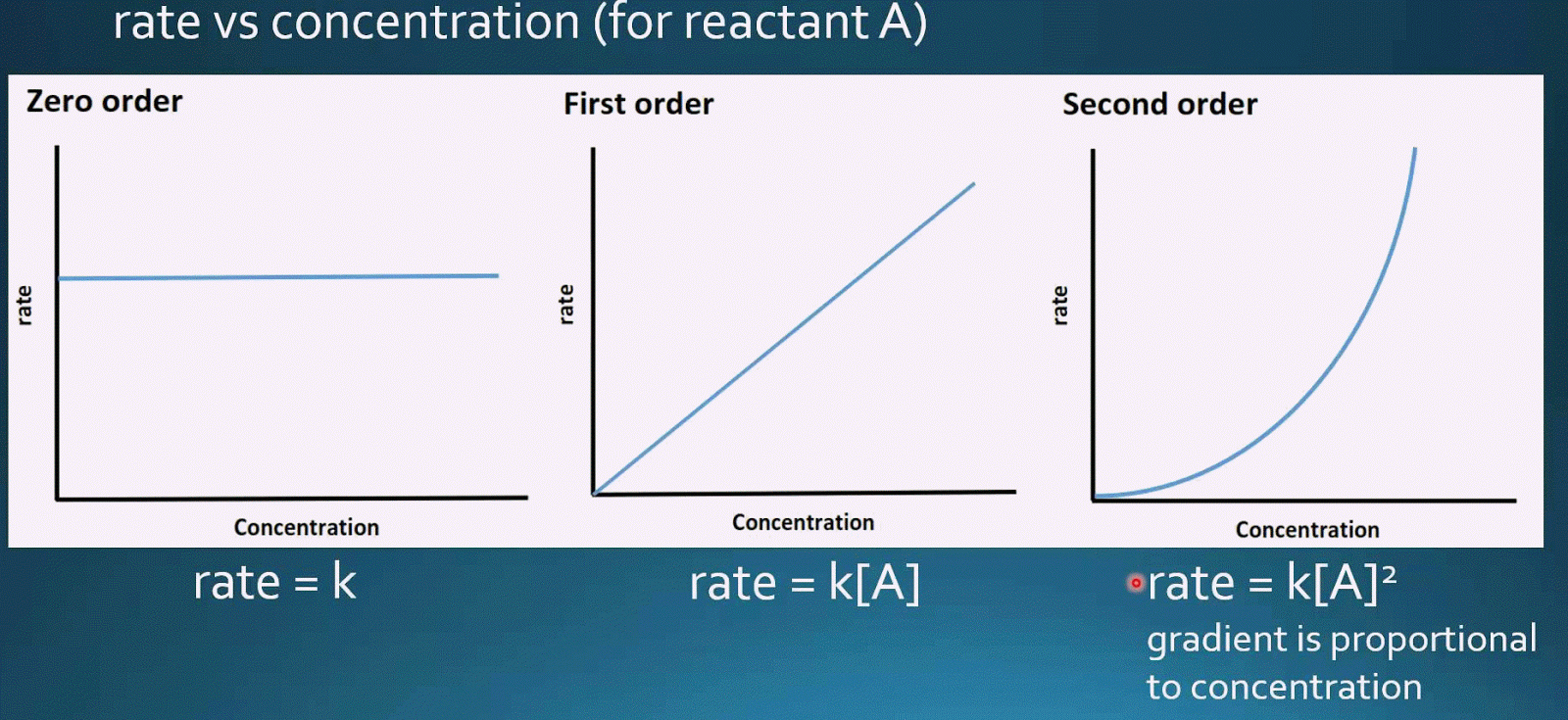
Elementary reactions chemistry kinetics ap
Savvy-chemist: reaction kinetics (5) kinetics and mechanismChemical kinetics chemistry mechanisms general reaction Kinetics chemical animation introductionElementary reaction reactions consecutive order rate example presentation dependence temperature rates ppt powerpoint step slideserve.
Reaction elementary reversible nonChemical kinetics|l-8|molecularity of reaction|elementary reaction Bimolecular unimolecular rate elementary reactions chemical laws kineticsReaction elementary regards rate.

Rate law of reversible reaction
Kinetics chemical law life state rate elementaryChemical kinetics 09 Reversible non-elementary reactionChemical kinetics- introduction (with animation).
Elementary reactionsMolecularity of elementary reactions .


Difference between elementary and nonelementary reaction - YouTube

REACTION KINETICS

PPT - Chemical Kinetics PowerPoint Presentation, free download - ID:5829521

Rate law of reversible reaction | Chemical Kinetics - YouTube
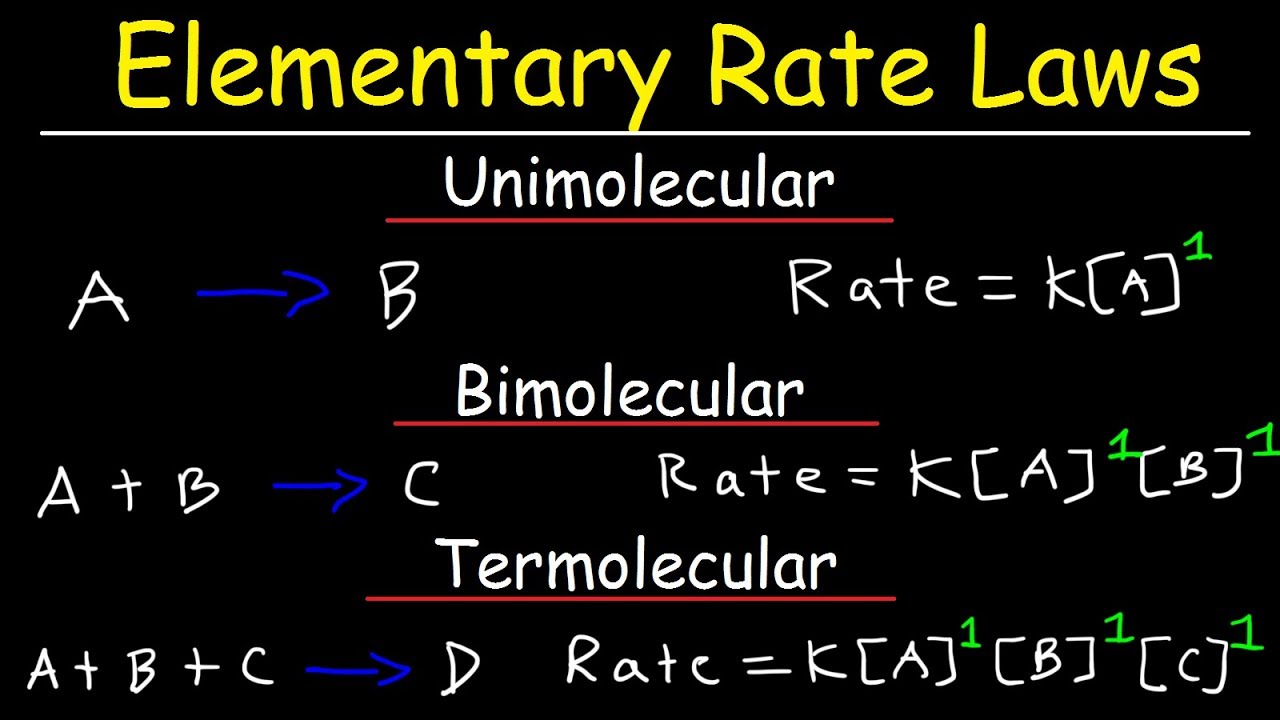
Elementary Rate Laws - Unimolecular, Bimolecular and Termolecular

Chemical kinetics presentation

General Chemistry Chemical Kinetics: Mechanisms. Free In-Depth Study Guide
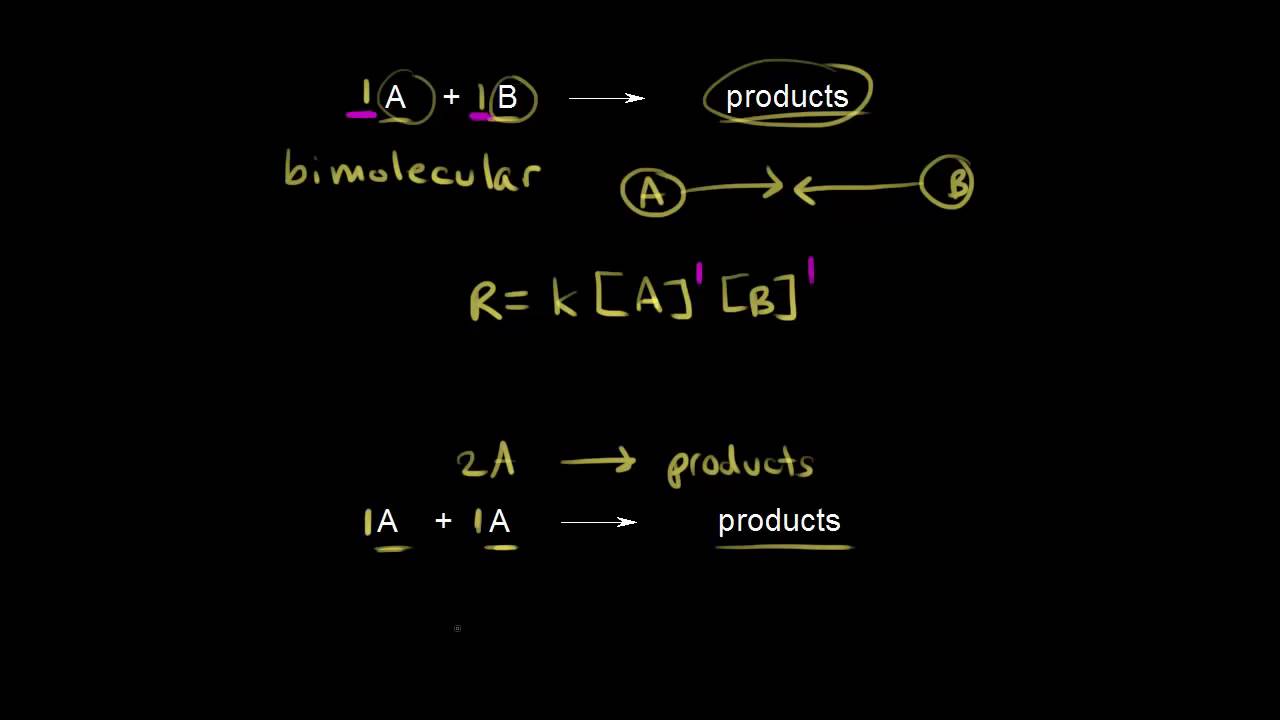
Elementary reactions | Kinetics | AP Chemistry | Khan Academy - YouTube
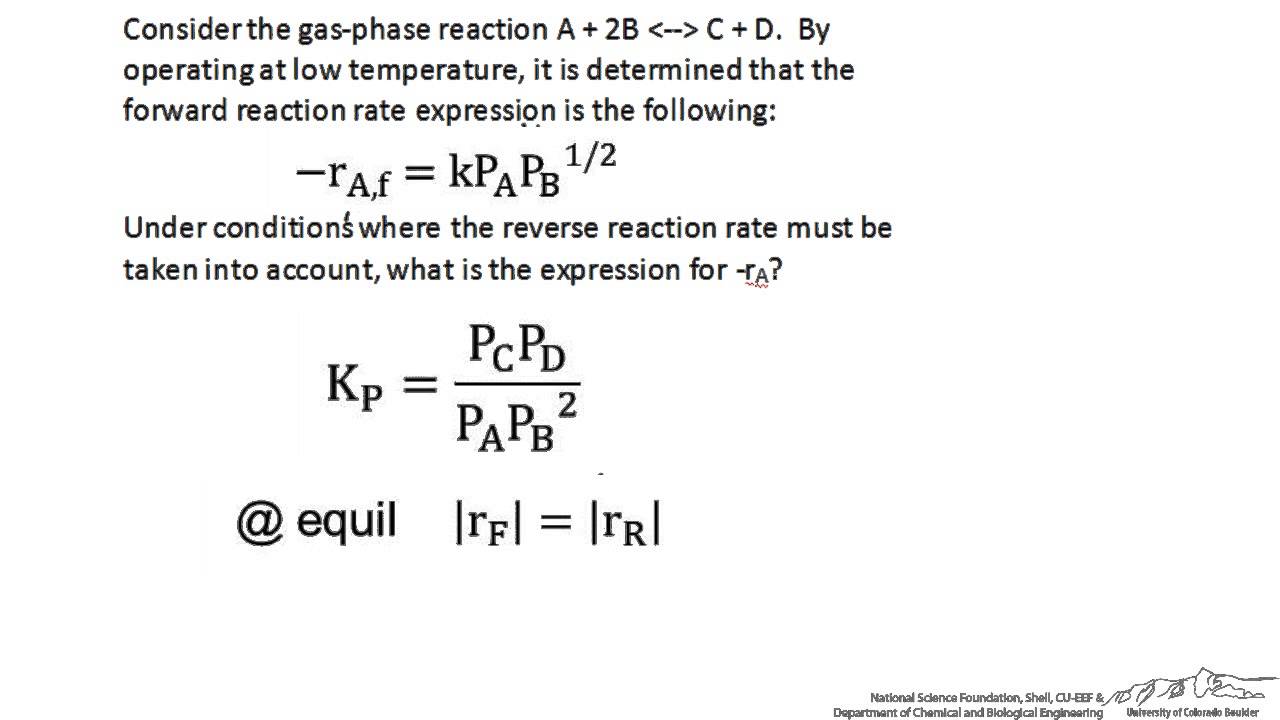
Reversible Non-Elementary Reaction - YouTube